Back to Top
Innovative technologies with groundbreaking potential in prostate cancer
Blue Earth Diagnostics is at the forefront in the investigation of novel radiopharmaceuticals to optimize their diagnostic capabilities and harness their therapeutic potential.
We have built on our strong foundations in developing and advancing radiopharmaceutical technology for positron emission tomography (PET) imaging in prostate cancer.
During PET imaging:
— 1 —
Radioisotopes are attached to a targeting molecule to show biologic processes, including metabolism, cellular proliferation, and receptor expression.1,2
— 2 —
Changes in biochemical activity may indicate disease activity at a molecular and cellular level.1
— 3 —
Compared with conventional imaging modalities such as computed tomography (CT), magnetic resonance imaging (MRI), and bone scan, PET imaging provides greater sensitivity and specificity.3
Radiohybrid™ agents are a new class of radiopharmaceutical with 2 isotope acceptor sites on 1 core molecule.4
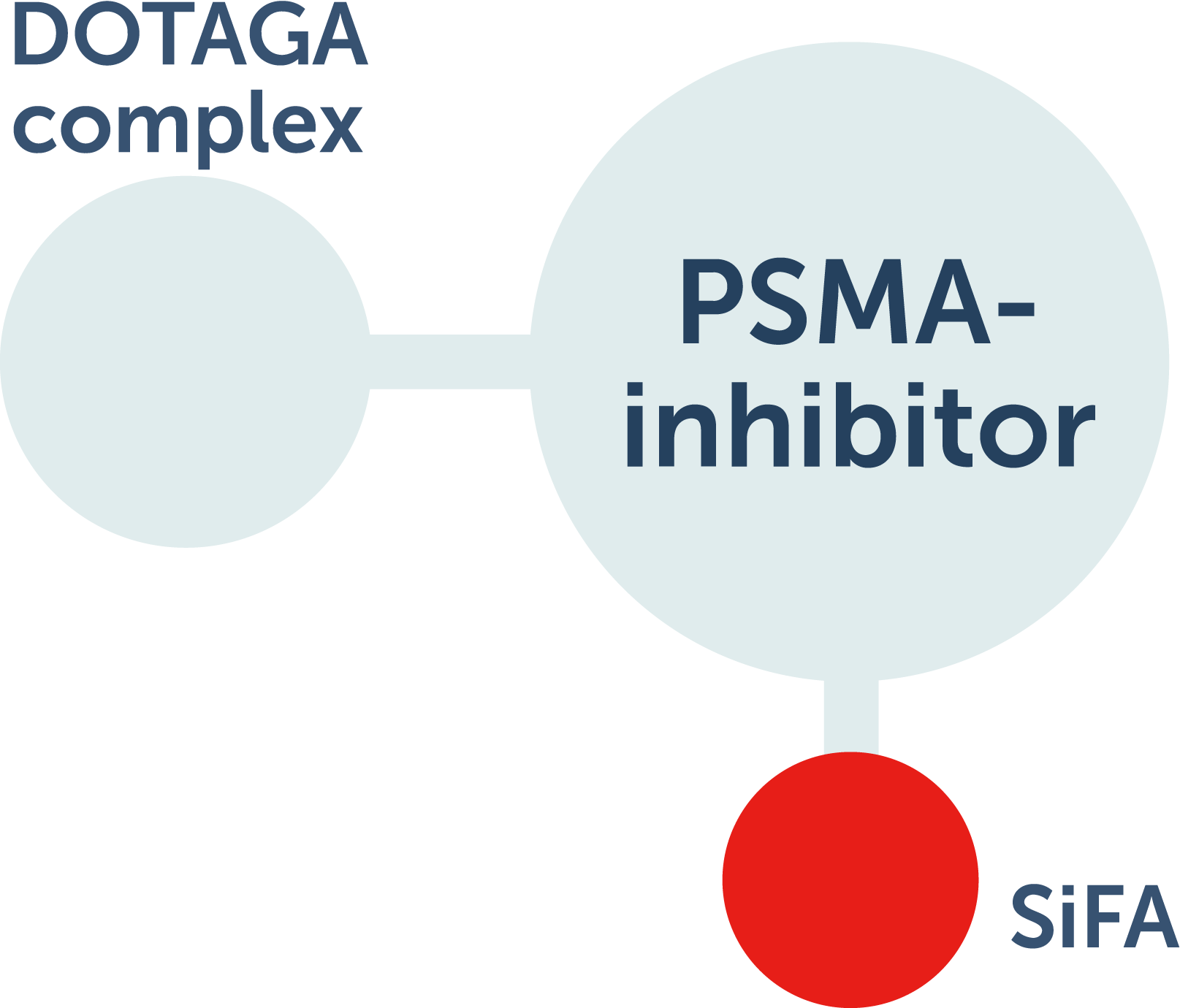
- Silicon-fluoride acceptor (SiFA) site can accept F 18 or F 19
- The DOTAGA complex in radiohybrid™ technology can be labeled with alpha- or beta-emitting radiometals or nonradioactive isotopes
For diagnostic use, 1 site is labeled with a radioactive imaging isotope and the other site with a nonradioactive isotope. For therapeutic use, the imaging isotope is nonradioactive and the other site is labeled with a therapeutic isotope. This potentially allows for theranostic (diagnostic and therapeutic) benefits, including localization of lesions, treatment planning and execution, and monitoring of treatment response.4,5
Currently available
How does POSLUMA work?
Hear Dr. Elizabeth Hawk, a specialist in nuclear medicine, explain POSLUMA’s radiohybrid™ molecular structure.
POSLUMA INDICATION
POSLUMA® (flotufolastat F 18) injection is indicated for positron emission tomography (PET) of prostate-specific membrane antigen (PSMA) positive lesions in men with prostate cancer
- with suspected metastasis who are candidates for initial definitive therapy
- with suspected recurrence based on elevated serum prostate-specific antigen (PSA) level
POSLUMA IMPORTANT SAFETY INFORMATION
- Image interpretation errors can occur with POSLUMA PET. A negative image does not rule out the presence of prostate cancer and a positive image does not confirm the presence of prostate cancer. The performance of POSLUMA for imaging metastatic pelvic lymph nodes in patients prior to initial definitive therapy seems to be affected by serum PSA levels and risk grouping. The performance of POSLUMA for imaging patients with biochemical evidence of recurrence of prostate cancer seems to be affected by serum PSA levels. Flotufolastat F 18 uptake is not specific for prostate cancer and may occur in other types of cancer, in non-malignant processes, and in normal tissues. Clinical correlation, which may include histopathological evaluation, is recommended.
- Risk of Image Misinterpretation in Patients with Suspected Prostate Cancer Recurrence: The interpretation of POSLUMA PET may differ depending on imaging readers, particularly in the prostate/prostate bed region. Because of the associated risk of false positive interpretation, consider multidisciplinary consultation and histopathological confirmation when clinical decision-making hinges on flotufolastat F 18 uptake only in the prostate/prostate bed region or only on uptake interpreted as borderline.
- POSLUMA use contributes to a patient’s overall long-term cumulative radiation exposure. Long-term cumulative radiation exposure is associated with an increased risk for cancer. Advise patients to hydrate before and after administration and to void frequently after administration. Ensure safe handling to minimize radiation exposure to the patient and health care providers.
- The adverse reactions reported in ≥0.4% of patients in clinical studies were diarrhea, blood pressure increase and injection site pain.
- Drug Interactions: androgen deprivation therapy (ADT) and other therapies targeting the androgen pathway, such as androgen receptor antagonists, may result in changes in uptake of flotufolastat F 18 in prostate cancer. The effect of these therapies on performance of POSLUMA PET has not been established.
To report suspected adverse reactions to POSLUMA, call 1-844-POSLUMA (1-844-767-5862) or contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Full POSLUMA prescribing information is available at www.posluma.com/prescribing-information.pdf
AXUMIN INDICATION
Axumin® (fluciclovine F 18) injection is indicated for positron emission tomography (PET) imaging in men with suspected prostate cancer recurrence based on elevated blood prostate specific antigen (PSA) levels following prior treatment.
AXUMIN IMPORTANT SAFETY INFORMATION
- Image interpretation errors can occur with Axumin PET imaging. A negative image does not rule out recurrent prostate cancer and a positive image does not confirm its presence. The performance of Axumin seems to be affected by PSA levels. Axumin uptake may occur with other cancers and benign prostatic hypertrophy in primary prostate cancer. Clinical correlation, which may include histopathological evaluation, is recommended.
- Hypersensitivity reactions, including anaphylaxis, may occur in patients who receive Axumin. Emergency resuscitation equipment and personnel should be immediately available.
- Axumin use contributes to a patient’s overall long-term cumulative radiation exposure, which is associated with an increased risk of cancer. Safe handling practices should be used to minimize radiation exposure to the patient and health care providers.
- Adverse reactions were reported in ≤1% of subjects during clinical studies with Axumin. The most common adverse reactions were injection site pain, injection site erythema and dysgeusia.
To report suspected adverse reactions to Axumin, call 1-855-AXUMIN1 (1-855-298-6461) or contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see Axumin full Prescribing Information.
Our pipeline
With worldwide, exclusive licenses to radiohybrid prostate-specific membrane antigen (rhPSMA) technology, we are continuing to investigate rhPSMA compounds for their potential use in diagnostic imaging. Our sister company, Blue Earth Therapeutics, is investigating their potential use as targeted radiopharmaceuticals for prostate cancer.
Explore Blue Earth Therapeutics' pipeline products.
References
- SNMMI. Fact Sheet: What is PET? 2016.
- SNMMI. About Radiopharmaceutical Therapies. Accessed October, 13, 2021. http://www.snmmi.org/Patients/About/content.aspx?ItemNumber=14792&navItemNumber=14793
- Crawford ED, Koo PJ, Shore N, et al. A clinician’s guide to next generation imaging in patients with advanced prostate cancer (RADAR III). J Urol. 2019;201(4):682-692.
- Wurzer A, Di Carlo D, Schmidt A, et al. Radiohybrid ligands: a novel tracer concept exemplified by 18F- or 68Ga-labeled rhPSMA inhibitors. J Nucl Med. 2020;61(5):735-742.
- Ballinger JR. Theranostic radiopharmaceuticals: established agents in current use. Br J Radio. 2018;91(1091):20170969. doi:10.1259/bjr.20170969